Week Two Questions
(Please enter your response below the question and be sure to include your
initials at the start of your response.)
In week two we are reading the paper by Cipra, "An Introduction to the Ising Model,"
American Mathematical Monthly, v. 94,10, pp. 937-959. The questions below are
designed to help you and your classmates understand the paper and begin thinking
about how the mathematical models of this paper might help answer the biological
questions raised in last week's reading. As per our class discussion of 2/22/08,
many of the questions below will still focus on our first reading. Future questions will
again return to the article on the Ising Model.
(For this week, let's try to read up through the first section of the Ising paper.)
Question #1 - Summarize the class discussion for 2/22/08. Be sure to highlight the
important points made in class. Give enough detail so that someone missing the class
could understand what was discussed.
(MM)
The class started out by discussing some technical issues with the course itself, which editor to use on the wiki, should point totals be posted for everyone to see, how many questions to aim to answer each week to stay on track to get a good grade... The point and click editor is the most user friendly, point totals will be posted and answering at least 2 questions each week should keep you on track, about 10 points a week. Now for the fun stuff...
We started out by Pete summarizing the beginning of the article and promptly being stopped and asked to explain a few words...Lipid, Bilayer, Leaflet, Protein and Domain. This pretty much took over the rest of the class period. Pete explained what a lipid is and what it is made up of. The fact that there are different lipids and how they are different was also discussed. The structure of a lipid, the fatty acid tail and functional group head, was also discussed along with how if it is saturated (all single bonds) or unsaturated (double and triple bonds) effects the tail shape.
From one lipid we progressed to multiple lipids and what they form...a lipid bilayer, in our case a phospholipid bilayer. The bilayer is composed of two "leaflets", which are rows of lipids. The leaflets face each other with their fatty acid tails (which are hydrophobic) facing each other and the functional group heads (hydrophilic) facing the outside.
Once we had assembled the lipid bilayer we discussed what the bilayer contained. The first topic that came up was a protein. A protein in a sequence of Amino Acids that are linked together by peptides (sometimes called peptide bonds). Proteins are made up of alpha helices and beta sheets. Proteins fold into specific shapes, where the shape determines its function. Theoretical Methods of determining how a protein will fold simply by its amino acid sequence do not exist, but are a major area of research currently.
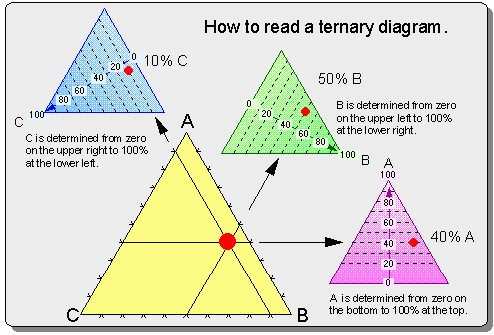
Next we moved to domains and got back into the article a little. The type of membranes that were being used in this experiment were discussed. They are artificial and contain only lipids. The type of lipids they used were lipids that were known to form vesicles in domains. A domain is a group of different lipids. The term asymmetric was thrown around for a while. We all tried to figure out what they were refering to when they called a leaflet asymmetric. Finally the answer that was agreed upon was that it meant the leaflets had different compositions of lipids which lead us to look at Figure 1, with the ternary phase diagram. I tried to explain how to read the diagram (see question #2). In this figure they show compositions of A B and C. We decided that each of these concentrations had the three lipids used in the experiment but in different concentrations.
We started to look at figure 2, which i suggested looked like a Punnet square and started to discuss what each picture looked like and how the experimental set up was. Then we ran out of time.
Question #2 - Let's check up on Meghan - explain the ternary phase diagram in Figure 1 of
Collins and Keller. Make sure you explain exactly how to read this diagram.
(MM) Is this directed for me to answer or someone else?
(JAP) Anyone can answer this one.
(KP) The ternary phase diagram will tell us the concentrations of cholesterol, DPPC, and DiPhyPC in each type of leaflet composition (A, B and C). The leftmost line segment represents the concentration of DiPhyPC. The rightmost line segment of the triangle represents the concetration of cholesterol and the bottom of the triangle is the concentration of DPPC. (Reading clockwise, the component should come first and then the axes that corresponds to it)To read the diagram first choose a point. Through that point you must draw 3 lines. Each line should be parallel to an axis of the triangle. Each vertex corresponds to the line drawn directly across from it. The point at which that line intersects the axes that corresponds to this vertex determines the concentration of that component. For example, if we look a point B we would discover that the concentrations of cholesterol, DPPC, and DiPhyPC are all exactly the same. In this experiment the concentration of cholesterol is constant, thus A, B, and C are all on the same horizontal line (directly across from the cholesterol vertex). As you move from left to right (A to C to B) the concentration of DiPhyPC increases in leaflet composition and the concentration of DPPC decreases.
(ZJ) This diagram helps show how to read ternary diagrams. In the Figure 1 of Collins and Keller, A would be cholesterol, B would be DPPC, and C would be DiPhyPC.
Question #3 - Explain the meaning of the term phase change.
(KP) A phase change is the transformation of a substance from one "phase" to another, where "phase" is the physical state (liquid, solid, or gas) of the substance. A phase change can be any of the following: liquid to solid (freezing), solid to liquid (melting), liquid to gas (boiling/evaporation), gas to liquid (condensation), solid to gas (sublimation) or gas to solid(deposition). Phase changes are caused by a change in temperature or pressure. The following is called a phase diagram and identifies the conditions at which different phase changes will occur. The critical point specifies the conditions at which the liquid state ceases to exist. The triple point specifies the conditions at which all three phases may coexist.

(AB) There is also another area in the phase diagram called the Super Critical Fluids. This is the region where the pressures and temperatures are greater then the critical point. In the SCF area, the liquid and gas become one fluid phase since at the critical point their densities are the same. It may be easier to think of SCF as they are in the real world: supercritical CO2 is used in the decaffeination of coffee and supercritical H2O is used to remove low concentration pollutants from wastewater. Below is a phase diagram showing the Super Critical Fluid region.

(ZJ) Phase changes involve changes in the energy of a system. When a phase change is not taking place, a change in energy leads to a change kinetic energy. This results in a change in temperature. During a phase change, a change in energy produces a change in potential energy because the distances between particles are changed. Because of this, there is no change in temperature or kinetic energy during a phase change, but there are changes in the physical properties of the material (wikipedia.org). As the graph below shows, at a constant pressure addition of heat energy at a constant rate produces plateaus in temperature.

Question #4 - Clearly explain Equation (1.1) from the Ising paper .
(AB) Before explaining the equation, I should say why they make the assumption that only “nearest-neighbor” interactions contribute to the energy level. They make this assumption because “nearest-neighbor” interactions give smaller, more workable numbers. On page 939 it says that there are 2N configurations. Therefore, if N were a large number, 2N would be a number that would be extremely too large to work with.
Here is the equation:
 ... I had to replace the greek symbol with an "s"
... I had to replace the greek symbol with an "s"
As for the equation itself:
H = total energy of the system
s = the value assigned to a specific lattice site
si and sj = the value of the spin at the specific lattice site, where s = +1 if the spin is pointing up or s = -1 if the spin is pointing down
It’s important to understand that for the first summation, the i and j in brackets (<i , j>) means that si*sj is added up over all possible nearest neighbor pairs. In the picture below, this would be the four possible red-yellow pairs.
Since the second summation is just for i, we can just add up si for lattice i.
Values E and J are both constants, where:
E = strength of the si and sj interaction
J = additional interaction of the individual spins with some external magnetic field
Furthermore, if E is positive, there is a low energy level and nearest-neighbor pairs have parallel moments (si = sj). This is referred to as ferromagnetism.
Also, the “external magnetic field” referred to in J, will tend to line up the magnetic moments in the direction of the field.
Here is a picture of a Simple Ising Model:

Question #5 - Explain the role of the partition function in statistical mechanics.
(MM)
The partition function is denoted by Z. It is "an important quantity that encodes the statistical properties of a system in thermodynamic equilibrium." (wikipedia.org). A system is said to be in thermodynamic equilibrium when the free energy is minimized. The partition function is a function of temperature and other parameters (microstates). This means that as temperature and microstate changes so will the partition function. A microstate is the configuration of a system as the system goes threw thermal fluctuations. There are different partition functions depending on the type of free energy that is used. One example of a partition function is called the Canonical partition function. A Canonical ensemble is a system that has a fixed volume, and number of constituent particles, as well as constant thermal contact with an environment, which has a temperature T. The canonical partition function is:

with

where
Kb is boltzmann's constant
T is temperature
Ej is the free energy of the jth microstate.
The partition function is a normalizing constant that makes sure that all the probabilities (states) add up to one. It "encodes how the probabilities are partitioned amound different microstates, based on their individual energies." (wikipedia.org). Most simply put, it is the measure of states that are available at a specific temperature.
Question #6 - I'd like to develop a convenient way to understand at a glance all of the results from the
Collins and Keller paper. Let's see what you can come up with.
(Pete) In short, Collins and Keller were interested in the effects of one leaflet (1 side of a lipid bilayer) on the other. Using artificial membranes that they constructed of 3 types of lipids, DiPhyPC, DPPC, and Cholesterol, they found that one leaflet can induce or suppress domain formation on the other. That is the lipid composition of one leaflet can cause the lipids in the complementary leaflet to either group together like lipids, or prevent them from grouping together. (I think this can be improved upon but I am stroggling to explain so much information in so few words. I will try again tomorrow.)
Question #7 - Brainstorm ways that the Ising model might be modified to help us understand the
bilayer problem.
(Pete) A possible way in which the Ising model may be modified to help understand the bilayer problem, would be to use a 2d lattice formation where we have the possible value of L(ordered) or L(disordered) phase at each vertex. The way in which it will drastically differ from magnetism model is that we must find a way to super-impose a second 2d lattice because it is the second leaflet that is inducing the domain formations, not the adjacent lipids.
(ZJ) To use the Ising model for the bilayer problem, we would have to determine the relationship between magnetism and polarity or some other energetic element of the lipid bilayers. We could modify the model to evaluate the energy changes associated with the phase transition temperatures of the different lipids in the leaflets. This may allow us to see if domains are formed based on the "favorable" free energy states as proposed by Collins and Keller.
Comments (0)
You don't have permission to comment on this page.